Accredited Investor Exclusive
Invest In
The First Gene Regeneration Therapy Yielding 300% Skin Regrowth
GENIE Therapeutics is pioneering aesthetic gene therapies. This breakthrough multi-drug Platform Technology, developed with Johns Hopkins and NIH funding, if FDA approved, has blockbuster potential.
Have questions? Call or text 302-405-2955
*This offering is being made under Regulation D 506c and is for accredited investors only. Learn more about our investor criteria here.
Accredited Investor Exclusive
Invest In The First Gene Regeneration Therapy Yielding 300% Skin Regrowth
GENIE Therapeutics is pioneering aesthetic gene therapies. This breakthrough multi-drug Platform Technology, developed with Johns Hopkins and NIH funding, if FDA approved, has blockbuster potential.
Have questions? Call or text 302-405-2955
*This offering is being made under Regulation D 506c and is for accredited investors only. Learn more about our investor criteria here.
Our Key Shareholders and NIH Research Funding
Johns Hopkins
Danaher
NIH
Our Key Shareholders and NIH Research Funding
Johns Hopkins
Danaher
NIH
Is This The Fountain
of Youth?
What if there were an actual fountain of youth? What if it delivered a new, true, non-invasive “age reversing” solution for wrinkles, sagging, under-eye bags and circles, baldness, and other high-anxiety skin issues?
Introducing The Gene Facelift™. If approved by the FDA, this is the easiest and most effective “Yes!” in anti-aging skin therapy to date for both doctors and patients. Read on to discover the economic upside potential this breakthrough multi-drug Platform Technology brings to the market.

Key Investment Highlights
Co-developed and co-patented with world-class researchers at Johns Hopkins and officially part of Johns Hopkins Technology Ventures, The Gene Facelift™ is a first-in-class drug candidate to potentially treat high-anxiety aesthetic issues such as wrinkles, under-eye bags and circles, baldness, rosacea, pigmentation, scarring, cellulite, and more.
Revolutionary Science
The company's patented gene therapy is the first of its kind potential solution that reverses skin aging, regenerates collagen, and significantly thickens skin - by a never before achieved 300% increase in preclinical studies - potentially eliminating wrinkles, under-eye bags, crow's feet, sagging skin, and other cosmetic concerns on the cellular level.
Institutional Support
Supported with NIH research funding¹ and guided by the company's team of ex-FDA veteran employee insiders to help advance FDA approval process steps. It is important to note that approvals for aesthetic drugs are generally less tedious than other drugs. In fact, we are not aware of an aesthetic drug candidate that was never successfully approved.
Powerful Partnerships
Danaher, ranked #132 on the Fortune 500 and part of the S&P 100, is a shareholder in GENIE Therapeutics. The company has formed a strong licensing partnership with Danaher for future high-yield HyperGRO™ GMP drug manufacturing. Danaher is highly qualified to meet all FDA GMP and CMC manufacturing requirements.
Platform Technology
GENIE Therapeutics has developed a multi-drug Platform Technology that may lead to multiple drug creations and launches from the same advanced DNA core, each one being a multibillion-dollar opportunity including wrinkles, hair loss, medical dermatology, and wound care.
$101 Billion Opportunity
Just three of the markets that GENIE Therapeutics is targeting (non-invasive aesthetics treatments, medical dermatology, and wound care) represent a total addressable market of $101 Billion.
Lower Barrier of Entry
Because The Gene Facelift™ is a topical aesthetic drug, the cost associated with potential FDA development and approval is estimated at ~$30 million, much lower than other drugs used systemically for diseases such as cancer or heart disease.
Key Investment Highlights
Co-developed and co-patented with world-class researchers at Johns Hopkins and officially part of Johns Hopkins Technology Ventures, The Gene Facelift™ is a first-in-class drug candidate to potentially treat high-anxiety aesthetic issues such as wrinkles, under-eye bags and circles, baldness, rosacea, pigmentation, scarring, cellulite, and more.
Revolutionary Science
The company's patented gene therapy is the first of its kind potential solution that reverses skin aging, regenerates collagen, and significantly thickens skin - by a never before achieved 300% increase in preclinical studies - potentially eliminating wrinkles, under-eye bags, crow's feet, sagging skin, and other cosmetic concerns on the cellular level.
Institutional Support
Supported with NIH research funding¹ and guided by the company's team of ex-FDA veteran employee insiders to help advance FDA approval process steps. It is important to note that approvals for aesthetic drugs are generally less tedious than other drugs. In fact, we are not aware of an aesthetic drug candidate that was never successfully approved.
Powerful Partnerships
Danaher, ranked #132 on the Fortune 500 and part of the S&P 100, is a shareholder in GENIE Therapeutics. The company has formed a strong licensing partnership with Danaher for future high-yield HyperGRO™ GMP drug manufacturing. Danaher is highly qualified to meet all FDA GMP and CMC manufacturing requirements.
Platform Technology
GENIE Therapeutics has developed a multi-drug Platform Technology that may lead to multiple drug creations and launches from the same advanced DNA core, each one being a multibillion-dollar opportunity including wrinkles, hair loss, medical dermatology, and wound care.
$101 Billion Opportunity
Just three of the markets that GENIE Therapeutics is targeting (non-invasive aesthetics treatments, medical dermatology, and wound care) represent a total addressable market of $101 Billion.
Lower Barrier of Entry
Because The Gene Facelift™ is a topical aesthetic drug, the cost associated with potential FDA development and approval is estimated at ~$30 million, much lower than other drugs used systemically for diseases such as cancer or heart disease.
The Problem
Lack of Innovation Has Created Tremendous Opportunity
A true regenerative solution for treating wrinkles and reversing skin age does not exist in the $61.2 billion per year global non-invasive aesthetics procedure market.²
No Pain No Gain
Toxins paralyze. Fillers plump. Other technologies ‘fry’ the skin. And most current options are invasive and painful.

No Innovation
A breakthrough anti-aging skin drug has not been developed in decades, leaving doctors and patients craving innovation.

Social Impact
In a social media comparative culture, anxiety and mental health issues over physical appearance comparison has increased.
The Problem
Lack of Innovation Has Created Tremendous Opportunity
A true regenerative cure for treating wrinkles and reversing skin age does not exist in the $61.2 billion per year global non-invasive aesthetics procedure market.²

No Pain No Gain
Toxins paralyze. Fillers plump. Other technologies ‘fry’ the skin. And most current options are invasive and painful.

No Innovation
A breakthrough anti-aging skin drug has not been developed in decades, leaving doctors and patients craving innovation.

Social Impact
In a social media comparative culture, anxiety and mental health issues over physical appearance comparison has increased.
The GENIE™ Therapeutics Multi-Drug Platform Technology Solution
Funded in part by the National Institutes of Health (NIH), The Gene Facelift™ is the first patented drug candidate to potentially regenerate aged, thin skin, reducing wrinkles, sagging, and other aesthetic and high-anxiety concerns.
Non-Invasive
The Gene Facelift™ is a non-invasive topical treatment of non-viral DNA. The topical application allows for ease of access and low patient burden.

Triples Skin Thickness
Preclinical data shows The Gene Facelift™ rapidly triples skin thickness, regenerating collagen, elastin, and skin stem cells.

High Profit Potential
The Gene Facelift™ is designed to be redosable, and could boost clinic profits by as much as 400% compared to current toxin treatments.
Funded in part by the National Institutes of Health (NIH), The Gene Facelift™ is the first patented drug candidate to potentially regenerate aged, thin skin, reducing wrinkles, sagging, and other aesthetic and high-anxiety concerns.
The GENIE Therapeutics Multi-Drug Platform Technology Solution

Non-Invasive
The Gene Facelift™ is a non-invasive topical treatment of non-viral DNA. The topical application allows for ease of access and low patient burden.

Triples Skin Thickness
Preclinical data shows The Gene Facelift™ rapidly triples skin thickness, regenerating collagen, elastin, and skin stem cells.

Highly Profitable
The Gene Facelift™ is designed to be redosable, and could boost clinic profits by as much as 400% compared to current toxin treatments.
The Optimized GENIE™ Multi-Drug Gene Therapy Platform Technology
Non-Viral GENIE
Platform Technology
Creation of multiple drugs from the same advanced DNA core platform
Minimal vector size for delivery of new multiple genes into cell
Minimalized CpG repeats for durability and high-protein expression
Antibiotic free
High-yield manufacturing
Novel Dermal Delivery System
Engineered with penetration enhancers
Evenly distributed with variable viscosity
Variable skin adhesive and mucoadhesive properties
Patented
Topical Gene Replacement Therapy
Redosable and titratable to achieve consistent expression
Off-the-shelf manufacturing and ease of administration
Patent protected gel formulation
Highly versatile addressing multiple indications
The Future of Genetic Aesthetic Medicine
Limited round access. Have questions?
Call or text 302-405-2955
The Optimized GENIE™ Multi-Drug Gene Therapy Platform Technology
Non-Viral GENIE
Platform Technology

Creation of multiple drugs from the same advanced DNA core platform
Minimal vector size for delivery of new genes into cells
Minimal CpG repeats for durability and high-protein expression
Antibiotic free
High-yield manufacturing
Novel Dermal Delivery System

Engineered with penetration enhancers
Evenly distributed with variable viscosity
Variable skin adhesive and mucoadhesive properties
Patented
Topical Gene Replacement Therapy

Redosable and titratable to achieve consistent expression
Off-the-shelf manufacturing and ease of administration
Patent protected gel formulation
Highly versatile addressing multiple indications
The Future of Genetic Aesthetic Medicine
Invest in GENIE Therapeutics today for $1 per share.
Limited round access. Have questions?
Call or text 302-405-2955
The DATA
GENIE’s Gene Facelift™ powers an unprecedented 300% increase in skin regeneration never previously scientifically achieved!
Preclinical Study #1
In a 4-day, single application study of GENIE Platform's GNE-142, epidermal thickness increased by 300%.
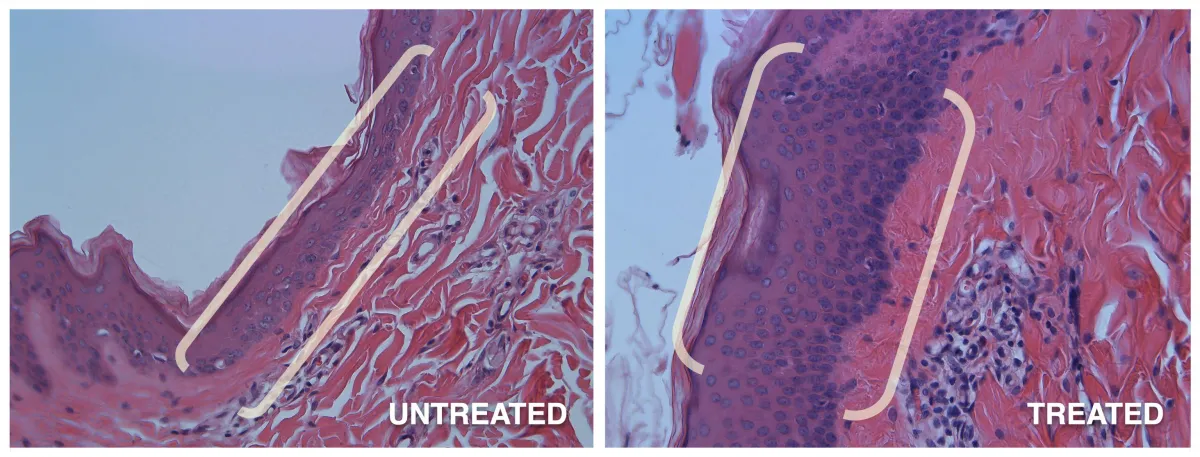
* Epidermis before and after.
In a 60-day, daily applied study of the #1 pharmaceutical non-drug skincare line, epidermal thickness minimally increased by only 27%.

Preclinical Study #2
In a 3-day, single application study of GENIE Platform’s GNE-142, dermal thickness increased by 300%.
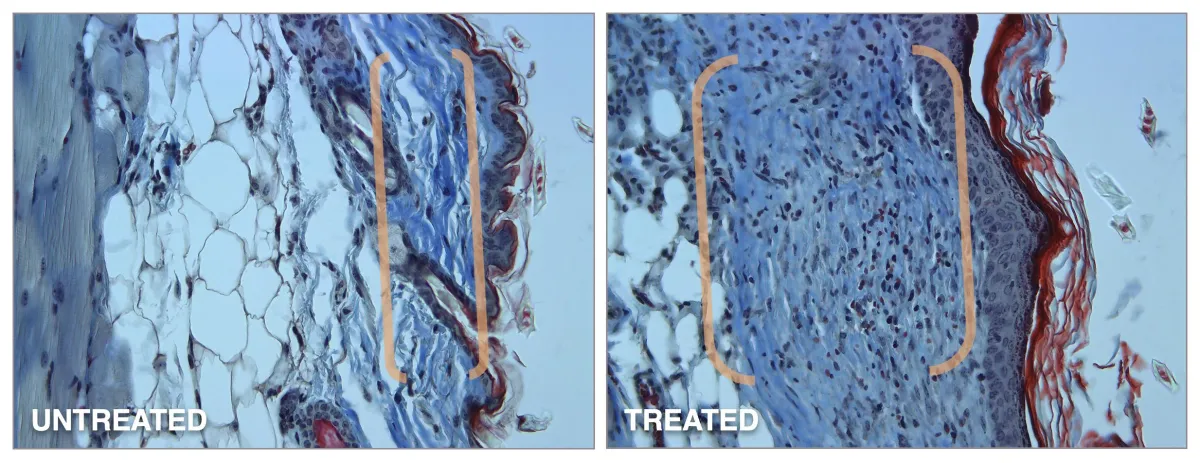
* Dermis before and after.
Single topical treatment shows significant regeneration of new elastin, collagen, stem cells, and skin thickness. Regenerated a 300% increase in skin thickness (12X greater than the #1 pharmaceutical non-drug skincare line).

Human Study #3
In a 4-day, single application study of GENIE Platform's GNE-142, massive amounts of new elastin was created in humans.
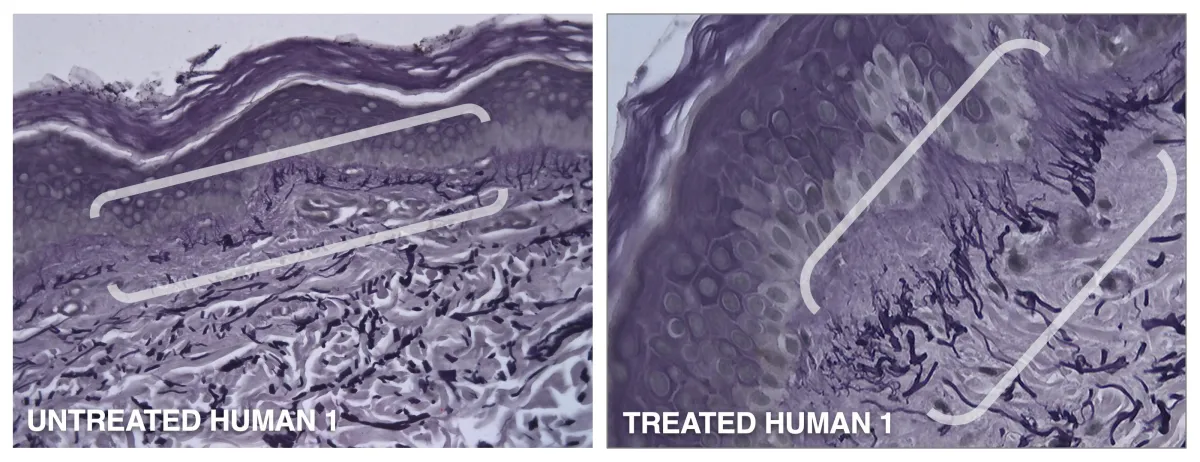
* Dermal-epidermal junction before and after.
Human in vivo subject #1, treated one time topically with GNE-142 shows new, massive organized elastin at the dermal-epidermal junction (DEJ) with perpendicular orientation and projections into epidermal stratum basale.
The DATA
GENIE’s Gene Facelift™ powers an unprecedented 300% increase in skin regeneration never previously scientifically achieved!

Study #1
In a 4-day, single application study of GENIE Platform's GNE-142, epidermal thickness increased by 300%.

* Epidermis before and after.
In a 60-day, daily applied study of the #1 pharmaceutical non-drug skincare line, epidermal thickness minimally increased by only 27%.

Study #2
In a 3-day, single application study of GENIE Platform’s GNE-142, dermal thickness increased by 300%.

* Dermis before and after.
Single topical treatment shows significant regeneration of new elastin, collagen, stem cells, and skin thickness. Regenerated a 300% increase in skin thickness (12X greater than the #1 pharmaceutical non-drug skincare line in 1/20th the time).

Human Study #3
In a 4-day, single application study of GENIE Platform's GNE-142, massive amounts of new elastin was created in humans.

* Dermal-epidermal junction before and after.
Human in vivo subject #1, treated one time topically with GNE-142 shows new, massive organized elastin at the dermal-epidermal junction (DEJ) with perpendicular orientation and projections into epidermal stratum basale.
Disrupting our way to a potential
$1+ billion in yearly sales
The GENIE multi-drug Platform Technology is capable of addressing numerous high-prevalence disease states within multiple billion-dollar markets.
Non-Invasive Aesthetics
$61.2B
Market²

Wound
Care
$20.8B
Market³
Medical Dermatology
$19.9B
Market⁴

Alopecia and Hair Loss
$8.2B
Market⁵
Disrupting our way to a potential
$1+ billion in yearly sales
The GENIE multi-drug Platform Technology is capable of addressing numerous high-prevalence disease states within multiple billion-dollar markets.

Non-Invasive Aesthetics
$61.2B
Market²

Wound
Care
$20.8B
Market³

Medical Dermatology
$19.9B
Market⁴

Alopecia and Hair Loss
$8.2B
Market⁵
GENIE Therapeutics Has Already Achieved Major Milestones
Since our inception, the team at GENIE has been hard at work creating breakthrough agreements and achieving major milestones.

GNE-142 target ID and validation
Johns Hopkins co-inventorship and exclusive license
Rational gel delivery design and multi-drug Platform Technology
NIH grant funding won
Proof of concept studies in mice
Proof of concept studies in swine
GMP / CMC started
US Patent issued
Angel capital received
Human in vivo skin proof of concept
Johns Hopkins Tech Ventures FastForward company designation achieved
Ex-Allergan VP joins company as shareholder and advisor
Series A round starts
Pathway to $1+ billion in yearly sales
Given the significant unmet market need, if approved by the FDA, GNE-142 has a clear runway to achieving $1+ billion in yearly sales.
Proven management team
High unmet needs, growing customer segments, global opportunity
Cash pay and buffered from political and reimbursement risks
Ability to partner with providers to drive rapid adoption
Attractive gross profits

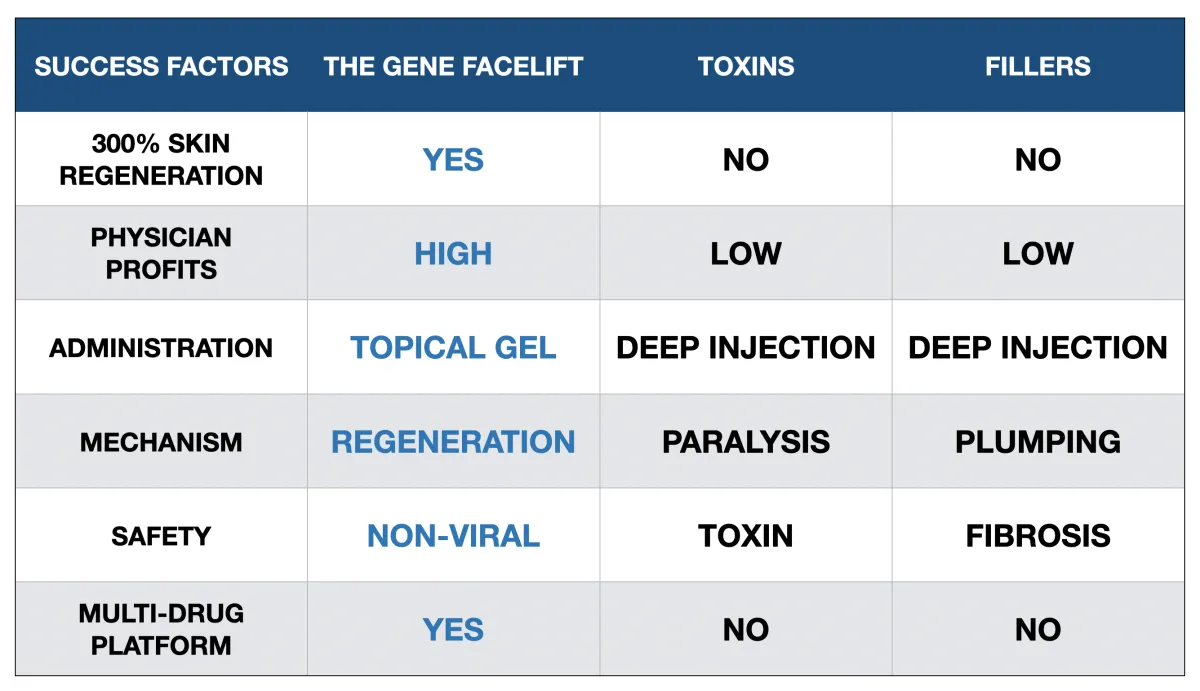
The GENIE Therapeutics Competitive Advantage
GENIE has a real competitive advantage over other cosmetic aesthetic treatments available today.
Pathway to $1+ billion in yearly sales
Given the significant unmet market need, if approved by the FDA, GNE-142 has a clear runway to achieving $1+ billion in yearly sales.
Proven management team
High unmet needs, growing customer segments, global opportunity
Cash pay and buffered from political and reimbursement risks
Ability to partner with providers to drive rapid adoption
Attractive gross profits


The GENIE Therapeutics Competitive Advantage
GENIE has a real potential competitive advantage over other aesthetic treatments available today.
The GENIE roadmap & clinical pipeline
GENIE Therapeutics has a catalyst-rich pipeline powered by our multi-drug Platform Technology with numerous value drivers pending FDA guidance. We are positioned to deliver significant value creation through numerous programs with near-term inflection points. Our potential timeline to commercialization:
YEAR 1
Start preclinical studies with ex-FDA experts
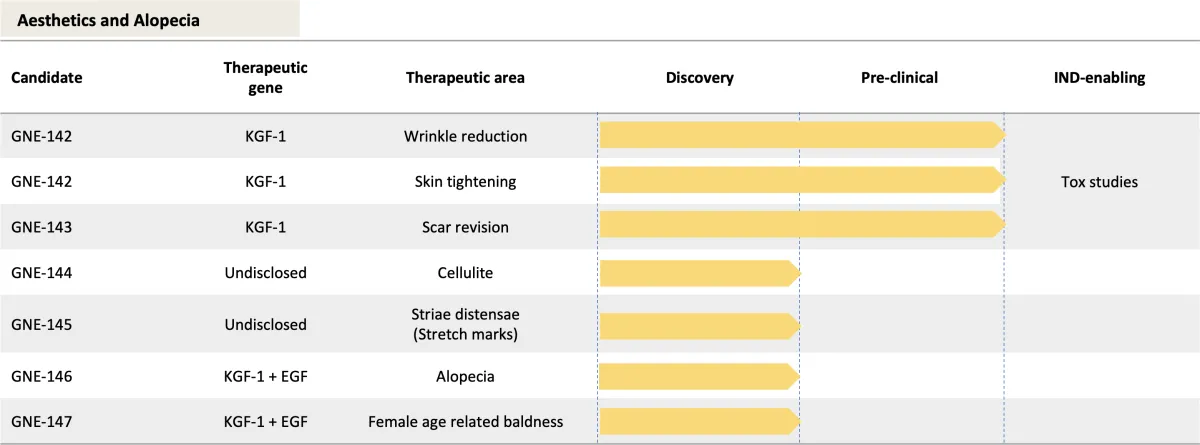
Submit IND with multiple indications: wrinkles, skin tightening, and scar revision (GNE-142, GNE-143)
Generate preclinical data on hair loss (GNE-146, GNE-147)
GMP / CMC process initiation
YEAR 2
Begin Phase 1/2 clinical studies on GNE-142, GNE-143
Submit IND on GNE-146, GNE-147
YEARS 3 & 4
Phase 1/2 readout on GNE-142, GNE-143
Begin Phase 3 studies on GNE-142, GNE-143
Begin Phase 1/2 clinical studies on GNE-146, GNE-147
YEAR 5
Submit BLA applications for GNE-142, GNE-143
Continue development and expansion of pipeline

The GENIE roadmap & clinical pipeline
GENIE Therapeutics has a catalyst-rich pipeline powered by our multi-drug Platform Technology with numerous value drivers pending FDA guidance. We are positioned to deliver significant value creation through numerous programs with near-term inflection points. Our potential timeline to commercialization:
YEAR 1
Start preclinical studies with ex-FDA experts
Submit IND with multiple indications: wrinkles, skin tightening, and scar revision (GNE-142, GNE-143)
Generate preclinical data on hair loss (GNE-146, GNE-147)
GMP / CMC process initiation
YEAR 2
Begin Phase 1/2 clinical studies on GNE-142, GNE-143
Submit IND on GNE-146, GNE-147
YEARS 3 & 4
Phase 1/2 readout on GNE-142, GNE-143
Begin Phase 3 studies on GNE-142, GNE-143
Begin Phase 1/2 clinical studies on GNE-146, GNE-147
YEAR 5
Submit BLA applications for GNE-142, GNE-143
Continue development and expansion of pipeline

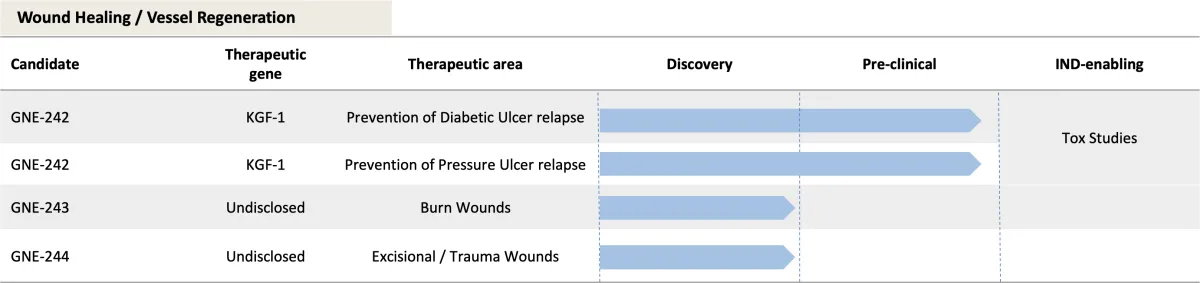
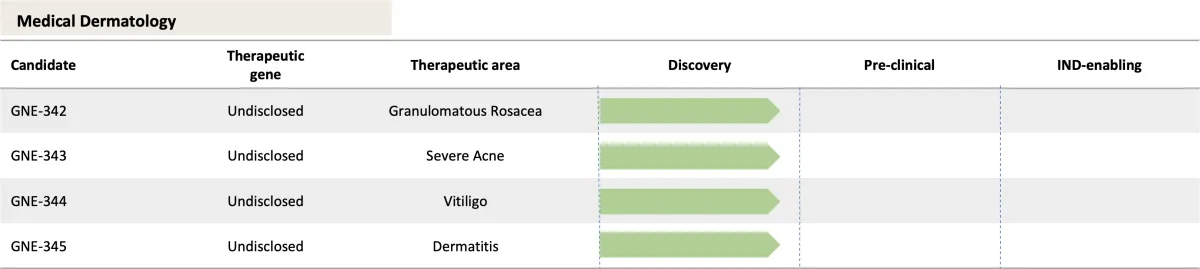
Clinical Pipeline
Because of our multi-drug Platform Techology, GENIE Therapeutics has a robust, multi-indication pipeline with several potential blockbusters.
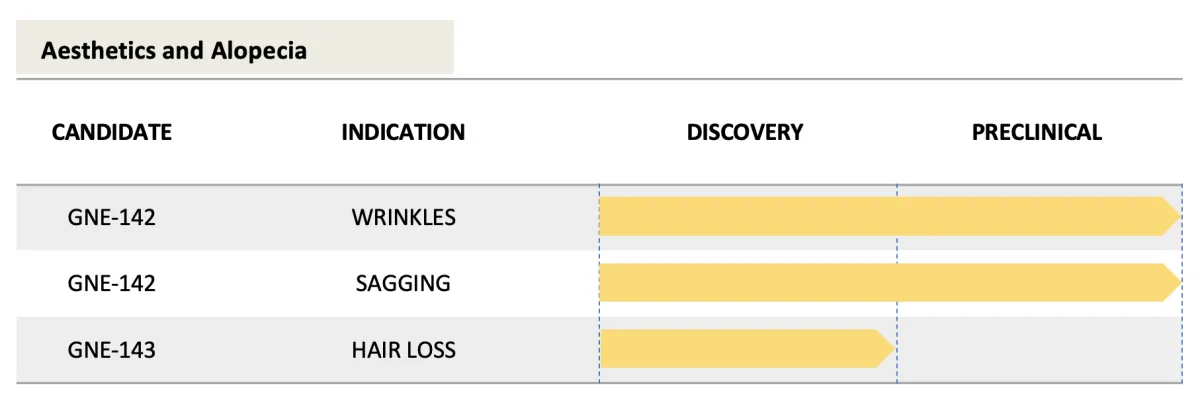
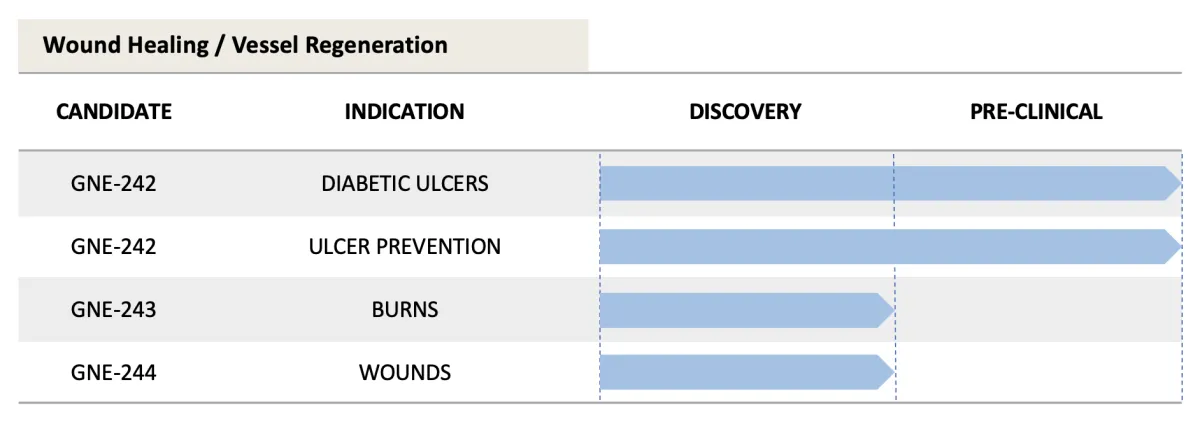
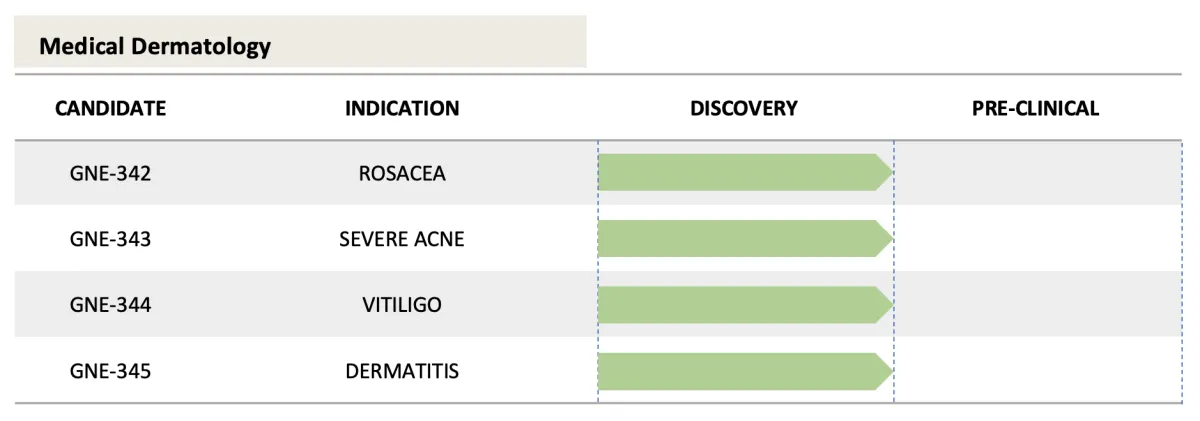
Clinical Pipeline
Because of our multi-drug Platform Technology, GENIE Therapeutics has a robust, multi-indication pipeline with several potential blockbusters.
Team Credentials
The GENIE team has extensive experience in aesthetics and dermatology, FDA regulatory approvals, and experience building businesses with exits. Our previous experience and interactions include the following companies, hospitals, and government institutes:






FDA
JOHNS HOPKINS
NIH
Team Credentials
The GENIE team has extensive experience in aesthetics and dermatology, FDA regulatory approvals, and experience building businesses with exits. Our previous experience and interactions include the following companies, hospitals, and government institutes:






FDA
JOHNS HOPKINS
NIH
Meet Our Expert Team

Aaron Tabor, MD
Founder & CEO
Aaron Tabor, MD, is the Founder, Co-Inventor and CEO of GENIE Therapeutics. Dr. Tabor is a graduate of the The Johns Hopkins School of Medicine. He is the lead inventor of GENIE Therapeutics’ “genetic cosmetics” First-in-Class aesthetic drugs. He has 20 years experience in recombinant DNA technology research, topical formulation and delivery science, and gene therapy drug development.

Joseph Pergolizzi, MD
Shareholder & Scientific Advisor
Joseph Pergolizzi, Jr., MD, is the prior Co-Director of Business Development for The Johns Hopkins Clinical Trials Unit (CTU). He is a former part-time Adjunct Assistant Professor at Johns Hopkins. Dr. Pergolizzi has extensive FDA experience as founder of NEMA Research, a Contract Research Organization (CRO) that has contributed to billions in drug and medical device sales.

Anish Mehta, MBA, MS
Shareholder & Advisor
Anish Mehta, a former VP of Allergan, is the founder and former CEO of Theramex, a $1 Billion global Women’s Health speciality pharmaceutical company. He has over 20+ years in the pharmaceutical industry, including a number of senior leadership roles with Allergan, Actavis and Baxter managing multiple $500m+ complex, global businesses. Currently, Anish serves as CEO of Synthon, an international pharmaceutical company that develops, manufactures and out-licenses medicines. MBA from The Wharton School.

Cassondra Todd
President
Cassondra Todd is an extraordinary marketer proven by an unparalleled track record of growing, directing and managing top-tier plastic surgery practices and providers in southern California. Her decades long series of successful achievements empowers her with deeply critical, multiple marketplace insights of both provider and patient needs to drive GENIE’s transformational venture in aesthetic and regenerative medicine. Cassondra’s understanding of both consumer and provider needs has solidified her as a trusted visionary in driving sales.

Bruce Schneider, MD
FDA Scientific Advisor
Ex-FDA Medical Officer employee (22+ years). Regulation of clinical trials of cellular and gene therapies including Aesthetic new drug applications. Graduate of Harvard Medical School. Prior Professor of Medicine at the Albert Einstein College of Medicine. Prior Chief, Division of Endocrinology and Metabolism Chief, Long Island Jewish Medical Center. Prior Assistant Professor, Rockefeller University.

Benjamin Vali, MS, RAC
FDA Scientific Advisor
Ex-FDA employee (13+ years) in Regulatory Affairs with focus on Regulatory Science and Pharmaceutical Product Development. Performed regulatory reviews of pre-IND and IND submissions, NDAs, and BLAs with a focus on the statistical aspects/methodology related to clinical trial design and data analysis in the evaluation of investigational drugs/biologics to inform medical decision making within the Office of New Drugs (OND).
The Future of Genetic Aesthetic Medicine
Invest in GENIE Therapeutics today for $1 per share.
Limited round access. Have questions?
Call or text 302-405-2955
The 5 Big Reasons To Consider Investing In GENIE Therapeutics

The GENIE Therapeutics Multi-Drug Platform Technology

One topical treatment shows significant regeneration of new elastin, collagen, stem cells, and skin thickness. GNE-142 regenerated a 300% increase in skin thickness – never before seen molecular age reversal results. This breakthrough Platform, developed with Johns Hopkins and NIH funding, has blockbuster potential to create multiple drugs from the same advanced DNA core. With revolutionizing aesthetics, it can be used across all full-body aesthetic skin conditions.

The Market Opportunity

The market potential is enormous, spanning both the aesthetic and medical; the ability to deliver additional drugs promises even greater returns. Over 16 million yearly US non-surgical cosmetic procedures generate $9 billion (Plastic Surgery Statistics Report: ASPS). Much of this revenue is derived from products and procedures with unsatisfactory efficacy and zero reverse aging and skin regeneration.
The Team Experience and Credentials

Our team is rock-solid, proven, and includes ex-FDA experts. We are leaders in medical research and FDA trial and approval. With drug approvals and sales under our belts, we collectively know how to take a drug through the FDA pipeline and to the finish line. Our key shareholders like Johns Hopkins and Danaher are global market leaders. Our previous experience and interactions include top-tier companies, hospitals, and government institutes.

The Rollout

The Gene Facelift™ will be restricted to doctor administration to complement toxin, filler, laser, or other treatments. Imagine asking your patients, “Would you like The Gene Facelift™ with your toxin today?” An easy, “Yes!” to increase your profits. In addition, doctors may earn potentially 400% more than toxin treatments. Patients are craving new, innovative treatments for their appearance. It’s for a true regenerative skin treatment.

The Exit Potential
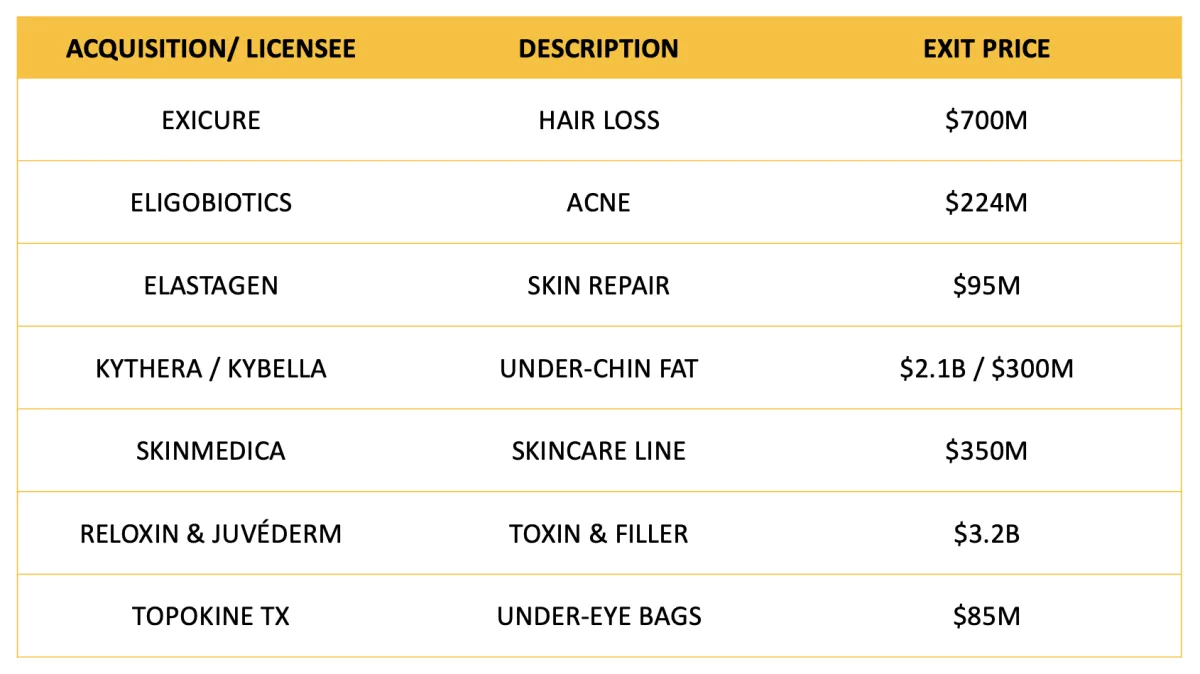
GENIE is uniquely positioned to execute against an extensive menu of full or partial potential exits. Each FDA milestone creates potential high demand for:
• Acquisition
• IPO
• Partnering
• Out-Licensing
A look at recent landmark deals demonstrates a strong demand to acquire aesthetic innovation.
The 5 Big Reasons To Consider Investing In GENIE Therapeutics


The GENIE Therapeutics Multi-Drug Platform Technology
One topical treatment shows significant regeneration of new elastin, collagen, stem cells, and skin thickness. GNE-142 regenerated a 300% increase in skin thickness – never before seen molecular age reversal results. This breakthrough Platform, developed with Johns Hopkins and NIH funding, has blockbuster potential to create multiple drugs from the same advanced DNA core. With revolutionizing aesthetics, it can be used across all full-body aesthetic skin conditions.

The Market Opportunity
The market potential is enormous, spanning both the aesthetic and medical; the ability to deliver additional drugs promises even greater returns. Over 16 million yearly US non-surgical cosmetic procedures generate $9 billion (Plastic Surgery Statistics Report: ASPS). Much of this revenue is derived from products and procedures with unsatisfactory efficacy and zero reverse aging and skin regeneration.



The Team Experience and Credentials
Our team is rock-solid, proven, and includes ex-FDA experts. We are leaders in medical research and FDA trial and approval. With drug approvals and sales under our belts, we collectively know how to take a drug through the FDA pipeline and to the finish line. Our key shareholders like Johns Hopkins and Danaher are global market leaders. Our previous experience and interactions include top-tier companies, hospitals, and government institutes.

The Rollout
The Gene Facelift™ will be restricted to doctor administration to complement toxin, filler, laser, or other treatments. Imagine asking your patients, “Would you like The Gene Facelift™ with your toxin today?” An easy, “Yes!” to increase your profits. In addition, doctors may earn potentially 400% more than toxin treatments. Patients are craving new, innovative treatments for their appearance. It’s for a true regenerative skin treatment.



The Exit Potential
GENIE is uniquely positioned to execute against an extensive menu of full or partial potential exits. Each FDA milestone creates potential high demand for:
• Acquisition
• IPO
• Partnering
• Out-Licensing
A look at recent landmark deals demonstrates a strong demand to acquire aesthetic innovation.
GENIE Therapeutics Offering Details
Join industry pioneer GENIE shareholders like Johns Hopkins and Danaher by investing in the new era of anti-aging skin regeneration and wound healing therapy.
Offering Type
Equity
Valuation Cap
$100M
Price Per Share
$1.00
Target Raise
$3M
Minimum Investment
$10,000
Exit Strategies
Acquisition, IPO, Partnering, and Out-Licensing
GENIE Therapeutics Offering Details
Join industry pioneer GENIE shareholders like Johns Hopkins and Danaher by investing in the new era of anti-aging skin regeneration and wound healing therapy.
Offering Type
Equity
Valuation Cap
$100M
Price Per Share
$1.00
Target Raise
$3M
Minimum Investment
$10,000
Exit Strategies
Acquisition, IPO, Partnering, and Out-Licensing
The Future of Genetic Aesthetic Medicine
Limited round access. Have questions?
Call or text 302-405-2955
The Future of Genetic Aesthetic Medicine
Limited round access. Have questions ?
Call or text 302-405-2955
Frequently Asked Questions
What is the purpose of GENIE Therapeutics equity fundraising campaign?
The purpose is to raise funds to develop The Gene Facelift™️ and other drugs and to pursue FDA clinical trials. We also intend to develop and launch a non-drug skin line using our Platform. Our next milestone is approximately $3 million for IND-enabling studies.
What is the minimum investment amount?
The minimum investment is $10,000 and there is no maximum amount.
When and how do I get my shares?
Shares are digitally recorded by our transfer agent VStock Transfer. Your Subscription Agreement is proof of your shares ownership.
Can I invest using a trust or an IRA or a Trust?
Yes. You can invest using an IRA or through a trust account. When going through the investment questionnaire, you will select your preferred method of investing. Contact us if you need assistance.
What is the time frame for GENIE Therapeutics to go public?
While there is no guarantee of going public, our strategy is to possibly go public within 18-36 months if we can achieve FDA milestones.
Will I have access to Gene Facelift and Genie Therapeutics products as an investor?
Yes! We plan if possible to give our investors early access to The Gene Facelift™ via medical tourism and other GENIE Therapeutics' products before the general public. Investors will also receive potential discounts (to be determined) on our products.
Is there a GENIE Therapeutics Data Room?
Yes. You can access the GENIE Therapeutics Data Room here.
What qualifications do I have to meet to be able to invest?
We are raising capital using a Reg D 506c exemption, which requires all investors to be accredited. An accredited investor is an individual with $200,000 in income for the past two years or a couple with a combined $300,000 in income or who has a $1M net worth excluding their primary residence. You may also qualify by holding certain licenses.
Why is your multi-drug Platform Technology important?
Our Platform Technology enables us to potentially create multiple drugs from the same advanced DNA core. This means we could address multiple aesthetic and wound healing indications.
Frequently Asked Questions
What is the purpose of GENIE Therapeutics equity fundraising campaign?
The purpose is to raise funds to develop The Gene Facelift™️ and other drugs and to pursue FDA clinical trials. We also intend to develop and launch a non-drug skin line using our Platform. Our next milestone is approximately $3 million for IND-enabling studies.

What is the minimum investment amount?
The minimum investment is $10,000 and there is no maximum amount.

When and how do I get my shares?
Shares are digitally recorded by our transfer agent VStock Transfer. Your Subscription Agreement is proof of your shares ownership.

Can I invest using a trust or an IRA or a Trust?
Yes. You can invest using an IRA or through a trust account. When going through the investment questionnaire, you will select your preferred method of investing. Contact us if you need assistance.

What is the time frame for GENIE Therapeutics to go public?
While there is no guarantee of going public, our strategy is to possibly go public within 18-36 months if we can achieve FDA milestones.

Will I have access to Gene Facelift and GENIE Therapeutics products as an investor?
Yes! We plan if possible to give our investors early access to The Gene Facelift™ via medical tourism and other GENIE Therapeutics' products before the general public. Investors will also receive potential discounts (to be determined) on our products.

Is there a GENIE Therapeutics Data Room?
Yes. You can access the GENIE Therapeutics Data Room here.

What qualifications do I have to meet to be able to invest?
We are raising capital using a Reg D 506c exemption, which requires all investors to be accredited. An accredited investor is an individual with $200,000 in income for the past two years or a couple with a combined $300,000 in income or who has a $1M net worth excluding their primary residence. You may also qualify by holding certain licenses.

Why is your multi-drug Platform Technology important?
Our Platform Technology enables us to potentially create multiple drugs from the same advanced DNA core. This means we could address multiple aesthetic and wound healing indications.


302-405-2955
Address: 1207 Delaware Ave, #1118
Wilmington, DE 19806
Legal Disclaimer and Forward-Looking Statements
This website contains predictive or “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of current or historical fact contained in this website, including statements that express our intentions, plans, objectives, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” “should,” “would” and similar expressions are intended to identify forward-looking statements. These statements are based on current expectations, estimates and projections made by management about our business, our industry and other conditions affecting our financial condition, results of operations or business prospects. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may differ materially from what is expressed or forecasted in, or implied by, the forward-looking statements due to numerous risks and uncertainties. Factors that could cause such outcomes and results to differ include, but are not limited to, risks and uncertainties arising from: our ability to raise sufficient capital to execute our business plan; expectations for the clinical and pre-clinical development, manufacturing, regulatory approval, and commercialization of our pharmaceutical product candidate or any other products we may acquire or in-license; our use of clinical research centers and other contractors; expectations for incurring capital expenditures to expand our research and development and manufacturing capabilities; expectations for generating revenue or becoming profitable on a sustained basis; expectations or ability to enter into marketing and other partnership agreements; expectations or ability to enter into product acquisition and in-licensing transactions; expectations or ability to build our own commercial infrastructure to manufacture, market and sell our product candidates; acceptance of our products by doctors, patients or payors; our ability to compete against other companies and research institutions; our ability to secure adequate protection for our intellectual property; our ability to attract and retain key personnel; availability of reimbursement for our products; expected losses; and expectations for future capital requirements. Any forward-looking statements speak only as of the date on which they are made, and we undertake no obligation to publicly update or revise any forward-looking statements to reflect events or circumstances that may arise thereafter, except as required by applicable law. Investors should evaluate any statements made by us in light of these important factors.
Sources:
1.National Institutes of Health (NIH) Small Business Innovation Research (SBIR) Johns Hopkins University Subcontract from SBIR R44 GM080768-02 phase 2
2.https://www.grandviewresearch.com/industry-analysis/non-invasive-aesthetic-treatment-market
3.https://finance.yahoo.com/news/wound-care-market-garner-27-113000122.html
4.https://www.alliedmarketresearch.com/dermatological-drugs-market
5.https://www.grandviewresearch.com/industry-analysis/alopecia-market

